The results of the research supported by the RakFond by Bulatov E.R.
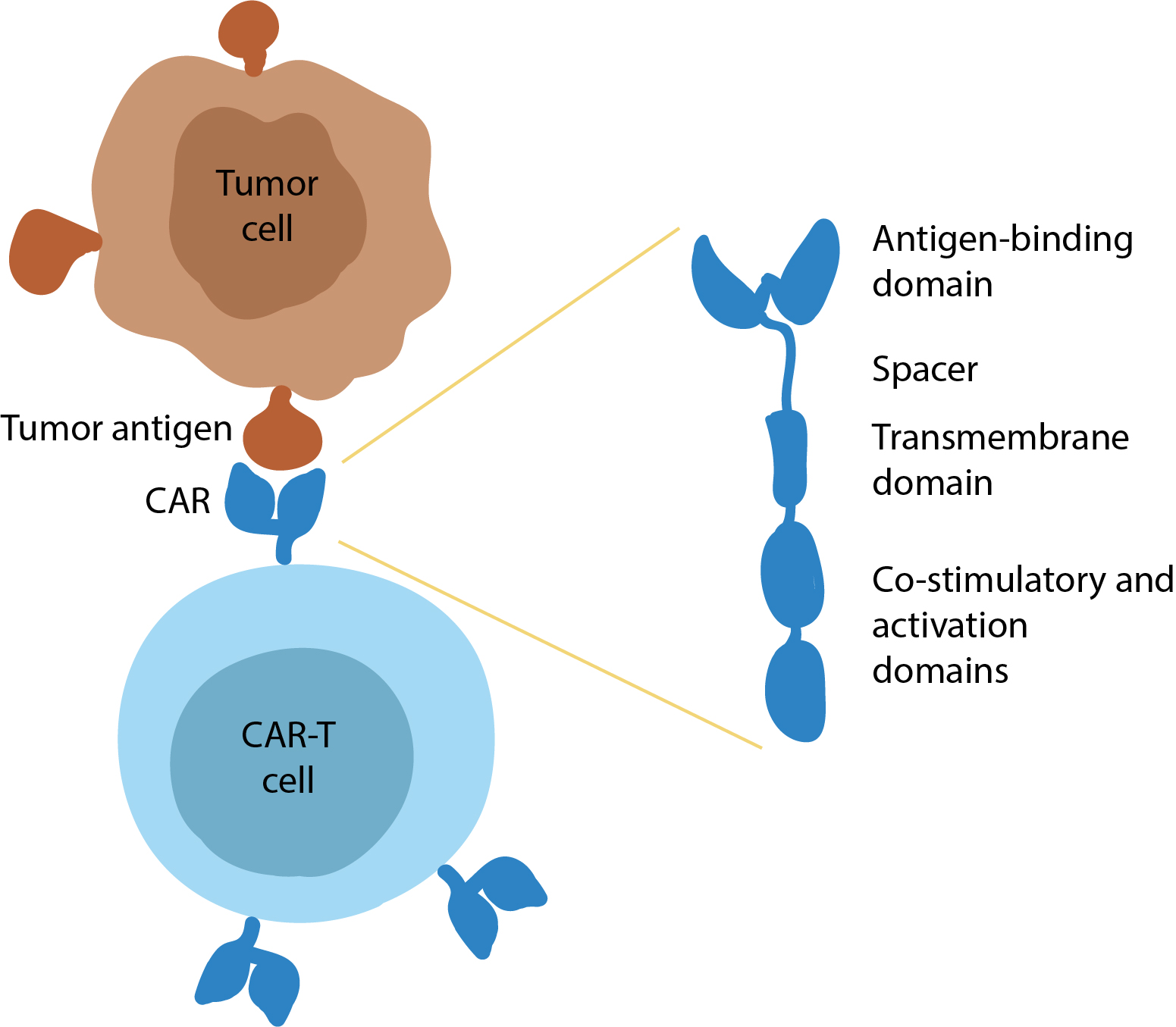
Immunotherapy with genetically modified lymphocytes, such as CAR-T cells, is one of the most promising areas of modern basic and clinical oncology. A CAR-T cell is a T-lymphocyte in which a gene encoding a CAR (chimeric antigen receptor) has been introduced ex vivo. Simplifying the concept, one can say that a T cell is a soldier of the regular troops, and a CAR-T cell is the special forces for performing highly complex tasks. An extracellular domain of the CAR provides recognition of the target tumor antigen (e.g. CD19), and an intracellular domain activates the mechanisms of T-cell cytotoxicity that result in destruction of the tumor cell.
The most common and well-characterized target is the pan-B-cell antigen CD19, which is expressed on the vast majority of malignant B cells. Currently, there are only two approved biomedical cell products for the treatment of hematological malignancies (Kymriah, Yescarta). However, despite the success of CAR-T cell therapy in the treatment of hematological malignancies, the application against solid tumors (carcinoma, neuroblastoma, etc.) remains an unresolved issue. This is due to several reasons, such as the high heterogeneity of cell composition of solid tumors, immunosuppressive tumor microenvironment, complicated penetration of CAR-T cells into the tumor stroma, local hypoxia, and the lack of nutrients inside the tumor. Given the high prevalence of solid tumors, finding ways to effectively use CAR-T immunotherapy for their treatment is extremely relevant.
Our project, funded by the Foundation for Cancer Research Support, is a part of a large study supported by the Russian Science Foundation and conducted jointly with the V.A. Almazov National Research Center (St. Petersburg). The main objective of the study is to elucidate the mechanisms of action of CAR-T cells in cellular and animal models of solid tumors.
We have already finished preliminary experiments on xenograft animal models with the orthotopic tumor, and the results are very encouraging. In our case, the data obtained in cell models of solid tumors were generally confirmed in subsequent experiments with immunodeficient mice. The CAR-T cells were non-toxic for mice and resulted in a marked reduction of the tumor size. I believe that our work will bring us closer to the development of domestic CAR-T cell products that will treat cancer patients and save their lives.
We are developing CAR-T cell products against solid tumors at the recently established Drug Discovery Center, and the first steps of translating our results into clinical practice will take place at the Clinical Research Center for Precision and Regenerative Medicine of Kazan Federal University.
Author: Bulatov E.R.